Biopsies are critical for accurate diagnosis and treatment. The chronic shortage of cancerous tissue available for analysis is a difficult obstacle to overcome in the field of oncology. Traditional biopsies typically only include one case per slide, which is oftentimes not enough tissue to complete a thorough analysis. Tissue microarrays (TMAs) are used to analyze gene expression in numerous pathological samples on one single slide.
Yale Pathology details the differences between conventional biopsies and tissue microarray services, highlighting the benefits of TMAs in both clinical and laboratory research settings.
Conventional Biopsy
One case per slide
Requires many batches for a single study
Requires a large amount of reagent
Subject to differential antigen retrieval
Very expensive and slow for large cohorts
150 sections of one case yield only 150 assays
Tissue Microarray
50-500+ cases per slide
An entire cohort can be done in a single experiment
Requires less than 1ml total reagent volume for the entire cohort
Not subject to differential antigen retrieval
Very rapid results, even when dealing with large cohorts
150 sections can have over 500 cases, yielding up to 75,000 assays.
Traditional Biopsy
A traditional biopsy is expensive and slow for large cohorts. They can only utilize one case per slide and require many batches for a single study, meaning it takes more resources and time to analyze the tissue available. Typically, medical professionals utilize needle biopsies at the time of diagnosis. A small amount of the tumor is removed, however, the needle typically does not remove enough tissue for a definitive diagnosis. In the field of oncology, a faster diagnosis means the patient can begin treatment sooner.
Why Use Tissue Microarrays?
Tissue microarrays are designed to study scarce tissue material to the fullest extent. Researchers can test hundreds of tissue samples with the antibodies of their choice, all at once. This reduces the amount of equipment, time, and space needed to study hundreds of tissue samples. Our customized tissue microarrays can be utilized to substantiate the importance of bio-targets in the advancement of diagnostics, therapeutics, and in considering new protein markers and genes.
How Does it Work?
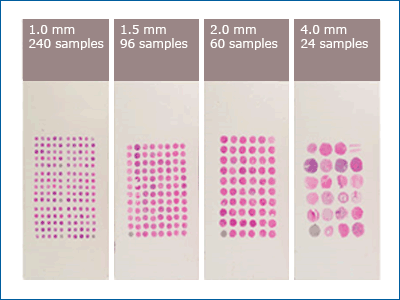
Currently, most tissue microarrays consist of paraffin-embedded tissues that are arranged into a paraffin block. The cancerous tissue is extracted from the subject by pricking the solid tissue with a circular needle and then sectioned onto the slide. The tissue is then preserved in the paraffin-embedded block. The needles can be sized around 0.6 mm to 4.0 mm, depending on how much tissue is available. The smaller the needle size, the more tissue samples can be collected.
Tissue microarray is proving to be an incredibly helpful technology in the field of science by increasing productivity, optimizing resources, and providing more accurate results.
Personalized Tissue Microarray Services
Tissue microarray is the future of diagnostics in oncology. We are proud to offer customized tissue microarrays. Our geneticists provide customized tissue microarrays for target definition or biomarker investigation. Our donors are carefully chosen. We utilize a massive biorepository of aliquots that allows for quick access and error-free shipments. Our biorepository services are IRB compliant and adhere to the highest industry standards, ensuring the best experience possible.
Purchase Your Tissue Microarray Now
Resources: